Burgmayer Lab
Welcome to the Burgmayer Lab . . . and the wonderful world of molybdenum!
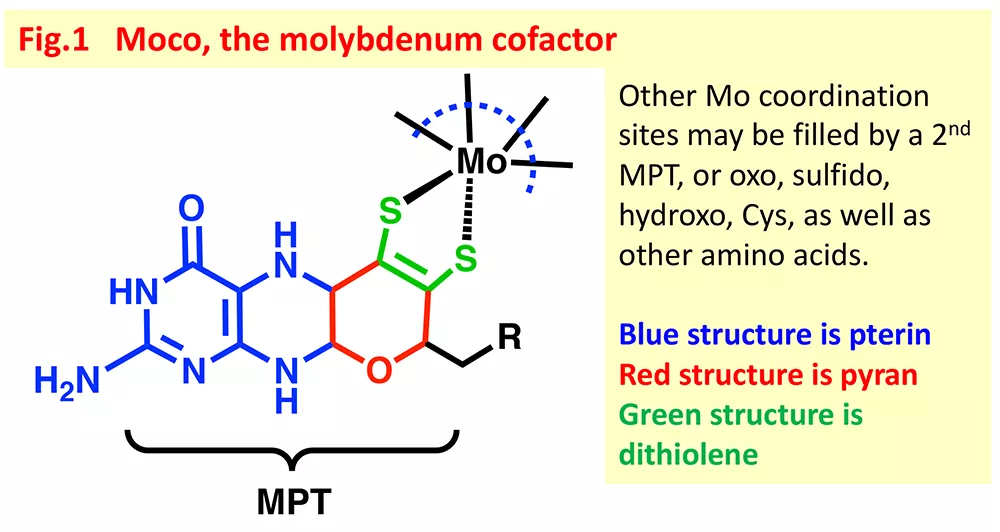
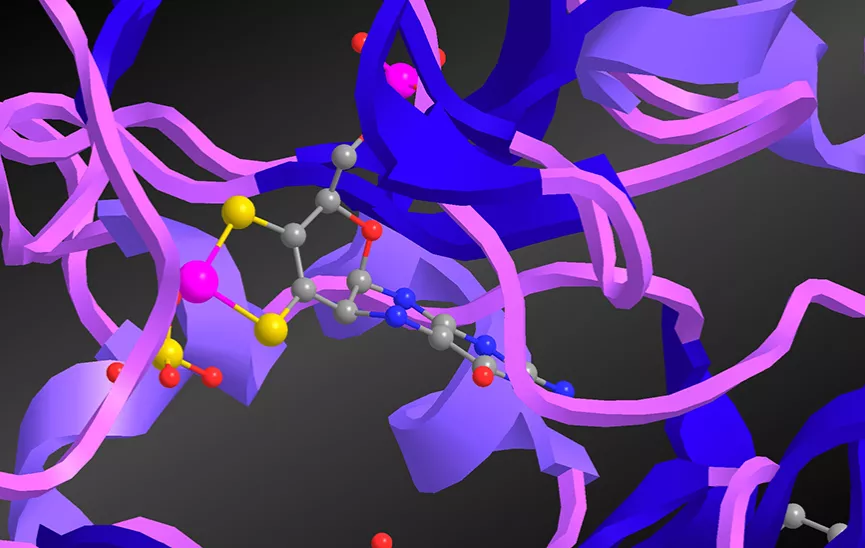
Molybdenum is a second row transition metal that is essential to your health, as well as to the health of almost every species on Earth. And this is because organisms use molybdenum enzymes to catalyze important biochemical reactions, such as respiration, protein synthesis and detoxification. Buried within the protein blankets of these Mo enzymes is the catalytic site known as the molybdenum cofactor, or Moco whose minimal composition is a Mo atom and a pterin-dithiolene ligand (Fig. 1). Our research focus is to understand what is special about the pterin-dithiolene ligand, and our approach is to study synthetic model compounds for Moco.
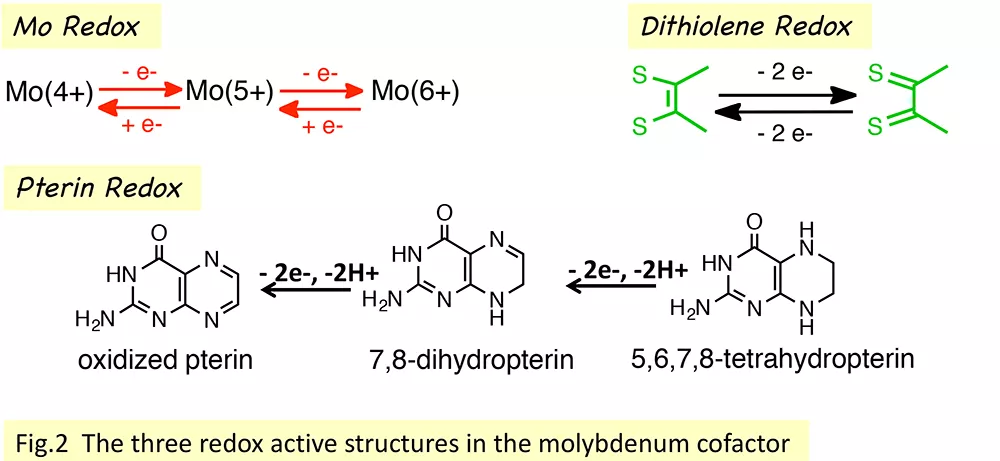
The pterin-dithiolene ligand—known as molybdopterin, MPT—that is found uniquely in molybdenum and tungsten enzymes continues to fascinate us. The complexity of this exceptional ligand has been postulated as key to the diversity of Mo and W enzyme function.1 The pterin dithiolene ligand has been increasingly appreciated as contributing a highly flexible electronic structure, not only because of the redox capability of both pterin and dithiolene, but also because of the role that pterin and pyranopterin conformation may play in adjusting the Mo reactivity. In fact, it is worth mentioning that Moco is the most redox rich cofactor in biology!
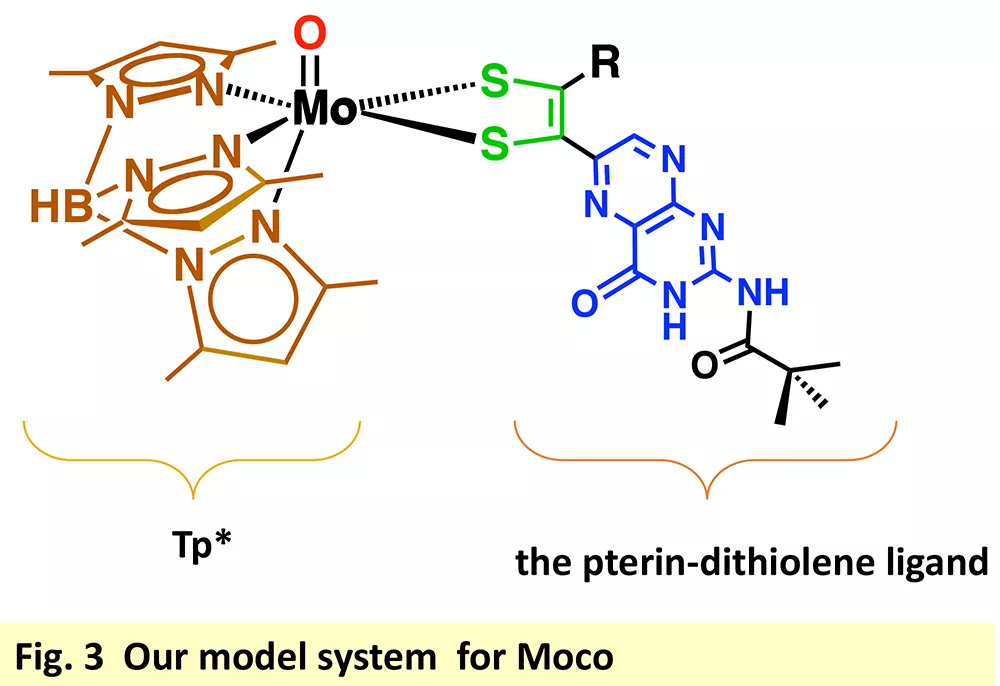
Our approach to the conundrum of the pterin dithiolene function has been to develop a model system that can be adjusted to explore a variety of questions pertinent to the enzymes. Our model system (Fig. 3) has allowed us to pursue several areas of exploration.
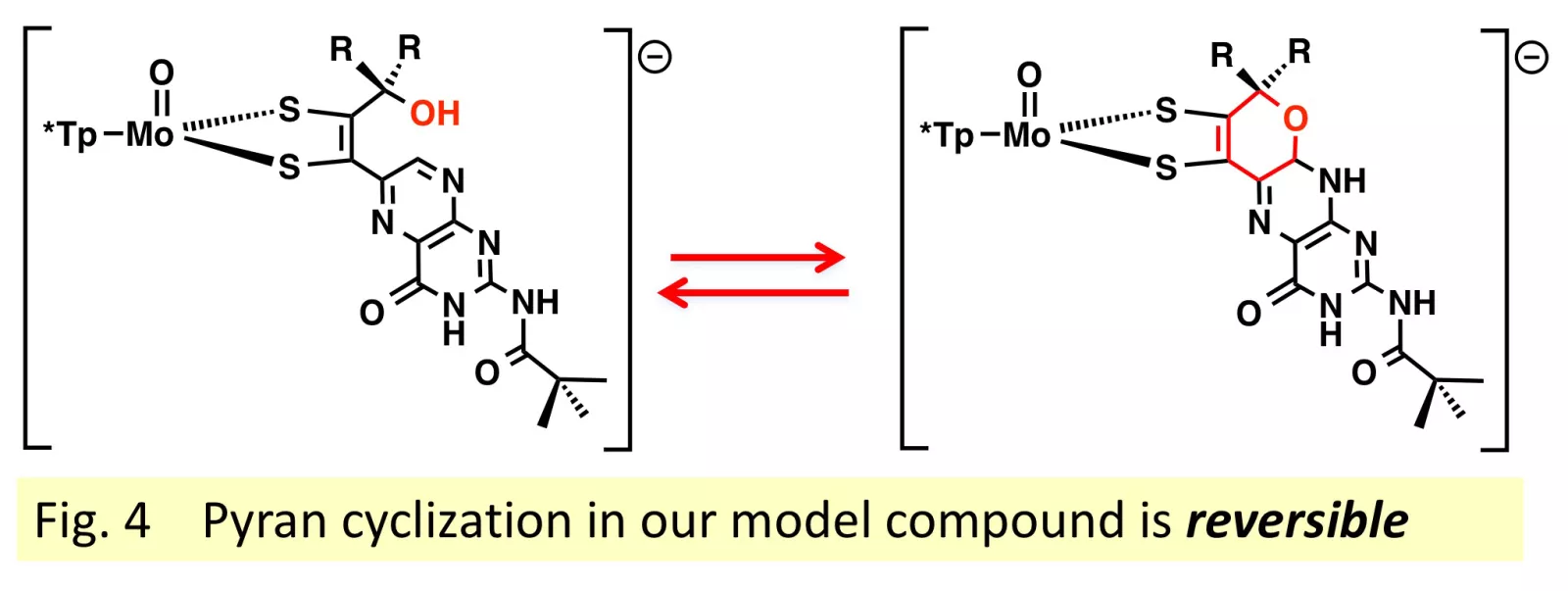
First, we have published2,3 our study of the reversible equilibrium between pyranopterin and uncyclized forms, and how that equilibrium shifts depending on solvent dielectric (or polarity). (Fig.4) We have determined this is a very low energy process.
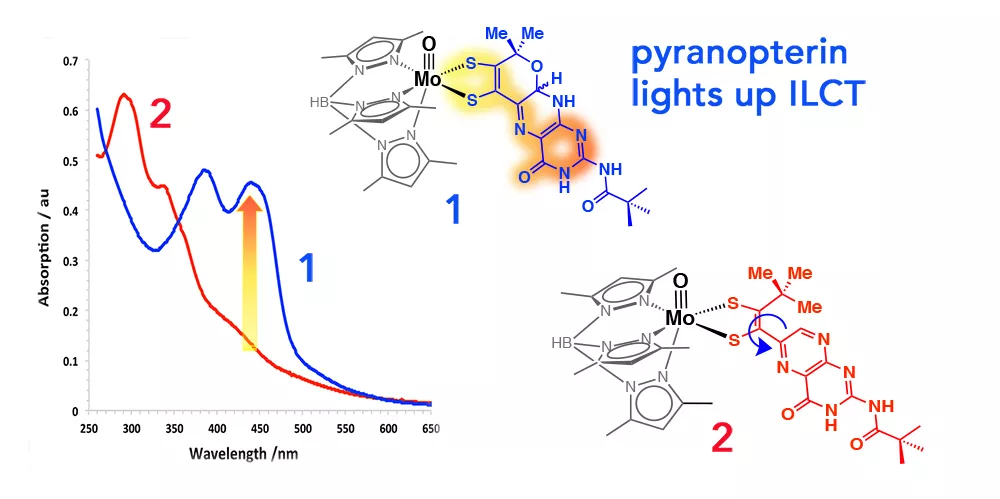
More recently, we have published a detailed study addressing the electronic consequences of pyranopterin formation (Fig. 5).4
Fig. 5. Intense ILCT occurs due to electronic delocalization in pterin-dithiolene ligand
Currently we are exploring the effects of protonating the pterin in our model, and the reduction of the pterin within the model. Together these results provide experimental evidence that the pyranopterin dithiolene ligand in molybdenum and tungsten enzymes could participate in many ways in catalysis through processes modulated by the protein. Our research has been supported by grants from NIH (GM081848) and NSF (CHE-0958996).
1 Rothery R, Weiner J, J Biol Inorg Chem, 2015, 349-372
2 Williams B R, Fu Y, Yap G P A, Burgmayer S J N, J. Am. Chem. Soc. 2012, 134, 19584−19587. DOI: 10.1021/ja310018e
3 Williams, B. R., Gisewhite, D., Kalinsky, A., Esmail, A., Burgmayer, S. J. N., Inorg. Chem. 2015, 54, 8214-8222. DOI: 10.1021/acs.inorgchem.5b00532
4Gisewhite, D., Yang, J.; Williams, B. R.; Esmail, A.; Stein, B.; Kirk, M.L.; Burgmayer, S. J. N. J. Am. Chem. Soc. 2018, 140, 12808-12818. DOI: 10.1021/jacs.8b05777

Contact Us
Department of Chemistry
Park Science Building
Bryn Mawr College
101 N. Merion Avenue
Bryn Mawr, Pennsylvania 19101
Phone: 610-526-7374
Fax: 610-526-5086